Human Factors I
Unlocking the Power of Human Factors in Medical Device Design
FOREWORD
Having navigated the complexities of Industrial Design and User Experience Design for medical devices during my enriching two years at Laerdal Medical, I'm thrilled to share my insights into the critical realm of Human Factors Engineering in medical device design. While many associate human factors with "ergonomics," in this blog, I won't focus on human capability or risk control. Instead, I'll discuss the three key aspects of human factors play in shaping the usability and safety of medical design.
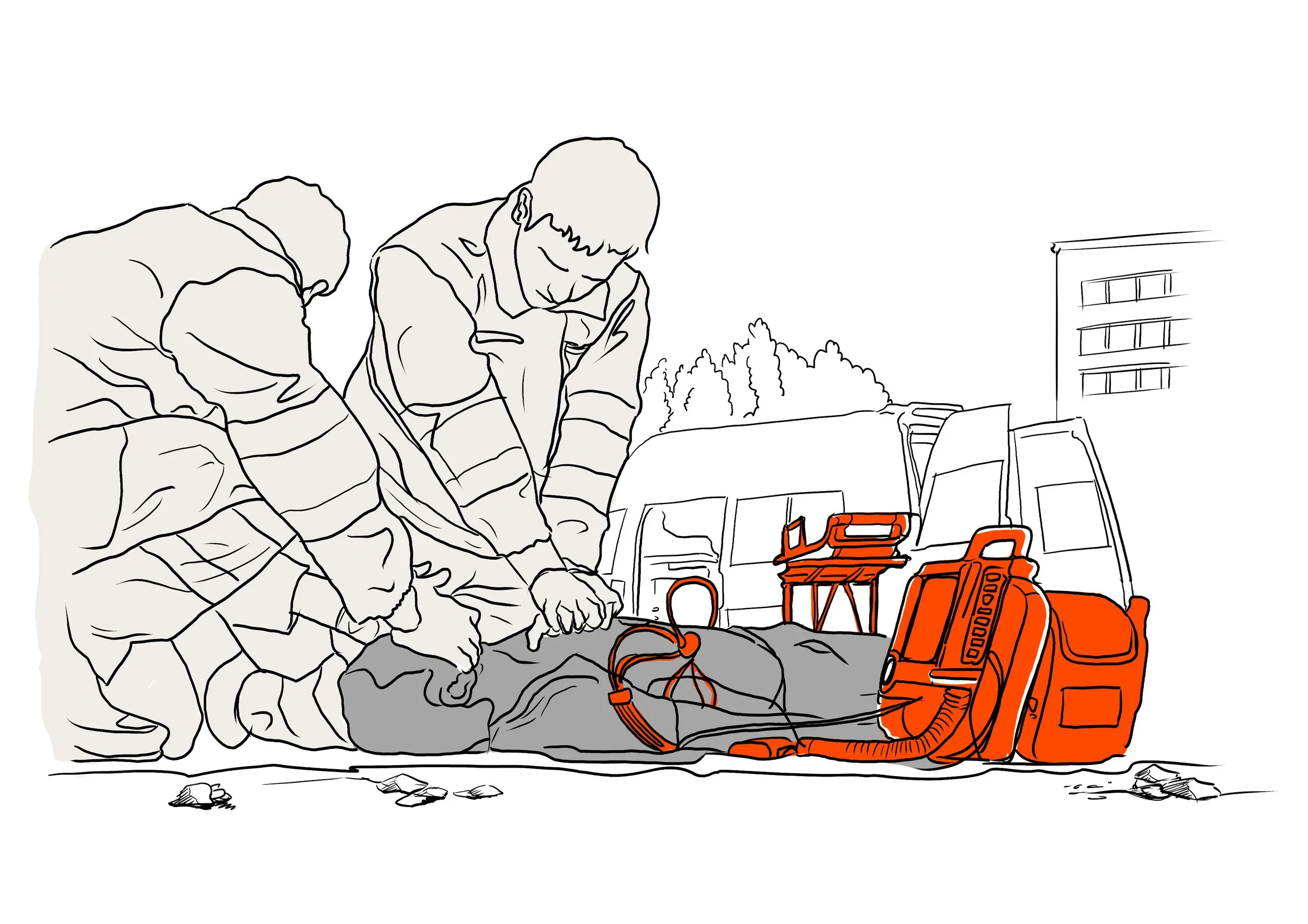
To kick off the definition of human factors in medical design, I've created an illustration that explains the three key aspects – Device User, User Interface and Device Environment.
In a cardiac arrest scene, the paramedics (Device User) administers CPR to the patient, simultaneously monitoring vital signs on the defibrillator's screen (User Interface) on the street next to the apartment (Device Environment).
WHAT IS HUMAN FACTORS
(Human Factor Engineering)?
The definition of human factors engineering by the Food and Drug Administration (FDA): the application of knowledge about human behavior, abilities, limitations, and other characteristics of medical device users to the design of medical devices including mechanical and software driven user interfaces, systems, tasks, user documentation, and user training to enhance and demonstrate safe and effective use. [1]
The motivation behind considering human factors is clear — to provide a new medical device that is safe and effective for the intended user, uses, and use environment.
To delve deeper into the common focus of design and human factors engineering, let's introduce the three key aspects that designers should be focused on based on human factors. These aspects form the backbone of a holistic approach, ensuring that medical devices not only meet regulatory standards but also align seamlessly with the needs and behaviours of end users.
01. Device Users
In the realm of human factors, the intended user refers to the end users primarily focused on – those who will use the device for patient medical care. For example:
EMTs (Emergency Medical Technicians) can be users of monitor and airway equipment, such as defibrillator and suction devices.
Neonatal nurses can be users of heart or cardiorespiratory monitors.
Additionally, it encompasses user characteristics and the expected level of user training, which I like to term a “user persona” for better clarity in understanding, especially for designers.
02. Device Environment
The healthcare environment is complex, varied, and tense, determining how the product should be designed to adapt to specific scenarios. It refers to the environment where end users will use the device. For instance:
Ambulances, patient homes, and outdoor environments can be where EMTs perform emergency treatment.
Neonatal intensive care units (NICU) or maternity wards can be where neonatal nurses resuscitate infants or relay important patient information.
Considering and evaluating the device environment is essential for medical device design. Lights, noise, environmental space, other medical equipment, humidity, etc., are factors that we, as designers, should holistically understand in the early stages of conceptualization. This understanding drives the reliability and ease of use for the device's user interface.
03. Device User Interface
For designers, it simply means the product design itself – all the interaction points on a device that the intended user has to interact with. This includes components and accessories, controls, visual displays, visual, auditory, and tactile feedback, alarms and alerts, logic and sequence of operation, labelling, and training.
Understanding all key aspects of human factors, I would like to share my experience of how it can be combined with industrial design for medical devices.
3 KEY ASPECTS IN MEDICAL DEVICE DESIGN
01. User Harmony - Crafting Designs Centered on People
Embarking on the design journey, putting users at the center is crucial for "people-centered design." I delved into qualitative research with more than 50 online interviews and four field trips in the UK, the US, and Norway. Exploring CPR training centers, medical device distributors, and hospitals greatly broadened my understanding of user needs during these two years.
Understanding end-user behavior here is like striking gold, offering feedback that's not just useful but also highly convincing. I've been human factors studies with 54 participants for a Class II medical device at Laerdal Medical, totaling around 3240 hours. These studies have been invaluable in grasping the intricacies of user behavior. Among them, 24 studies focused on validating the early design (Human factors formative study), and 30 were aimed at the final validation of the device (Human factors summative study). From paramedics and EMTs to emergency nurses, respiratory therapists, and anesthesiologists, alongside homecare laypersons, each group exhibited unique behaviors that influenced my design considerations. Recognizing that many use errors can be prevented from the start by truly understanding end users underscores the method to the madness.
Creating medical devices that users love requires stepping into their shoes. Dive into their world by understanding workflows, daily routines, and crafting journey maps and storyboards. Ensure your device seamlessly integrates into their routine, simplifying their day.
02. Beyond the Device - Navigating Environments for Seamless Integration
Let's not overlook this - the environment where the device operates is crucial. To understand the workflow details, it's essential to know where your device is used. Field trips revealed the time constraints and specific locations where healthcare professionals operate. Elements like lighting and background noise can be significant distractions. And, keep in mind, your device can also divert attention. It's not only about the medical equipment; it's always about the patient. Regardless of the information your device's interface provides, the patient remains the primary focus.
03. Interface Alchemy - Crafting Designs that Click with Users
Now, here's the spotlight - the device user interface. We, designers, often have our eyes glued here. With insights into end users and their world, let's scrutinize if the interface is their BFF. Ask yourself:
Is the device switch easily operable with medical gloves?
Does the device display information in harmony with patient care tasks?
Are LEDs, labels, and buttons visible in both bright (hospital patient room) and dark (midnight outdoor) conditions?
Is it practical to include an alarm in a noisy environment?
How easy is it for the healthcare team to clean and disinfect the reusable part of the device?
As we conclude our exploration into these three pivotal aspects of human factors for designers, allow me to share a closing story: in our usability testing for a medical device I'm involved with, we found that the initial concern about operation noise distracting users was unfounded. Surprisingly, users became accustomed to the sound, finding comfort in its familiarity. Strikingly, a quiet and efficient machine triggered unease, with one user stating, "I thought it's a prototype and shut down. But it still works, which could stress me out in an emergency."
CONCLUSION
It is essential to learn, understand, and design a medical device across the three key aspects not only during the medical design validation phase (Human Factors study). At least from my experience, it is a key at the beginning of the design conceptualization. We know that it is impossible to design a solution that the intended user never makes a user error through the medical device, but it is always a good starting point to keep in mind the human factors approaches throughout the whole product development as a medical designer. This is the golden rule of medical device design - be safe and effective for the intended users, uses, and use environments!
To achieve this, risk control has to be considered, which I would like to share more experience in my next article - Medical Design - Human Factors II.
References
Center for Devices and Radiological Health. “Applying Human Factors and Usability Engineering to Medical Devices.” U.S. Food And Drug Administration, 6 Sept. 2018, www.fda.gov/regulatory-information/search-fda-guidance-documents/applying-human-factors-and-usability-engineering-medical-devices.